How does the crystallization process occur?
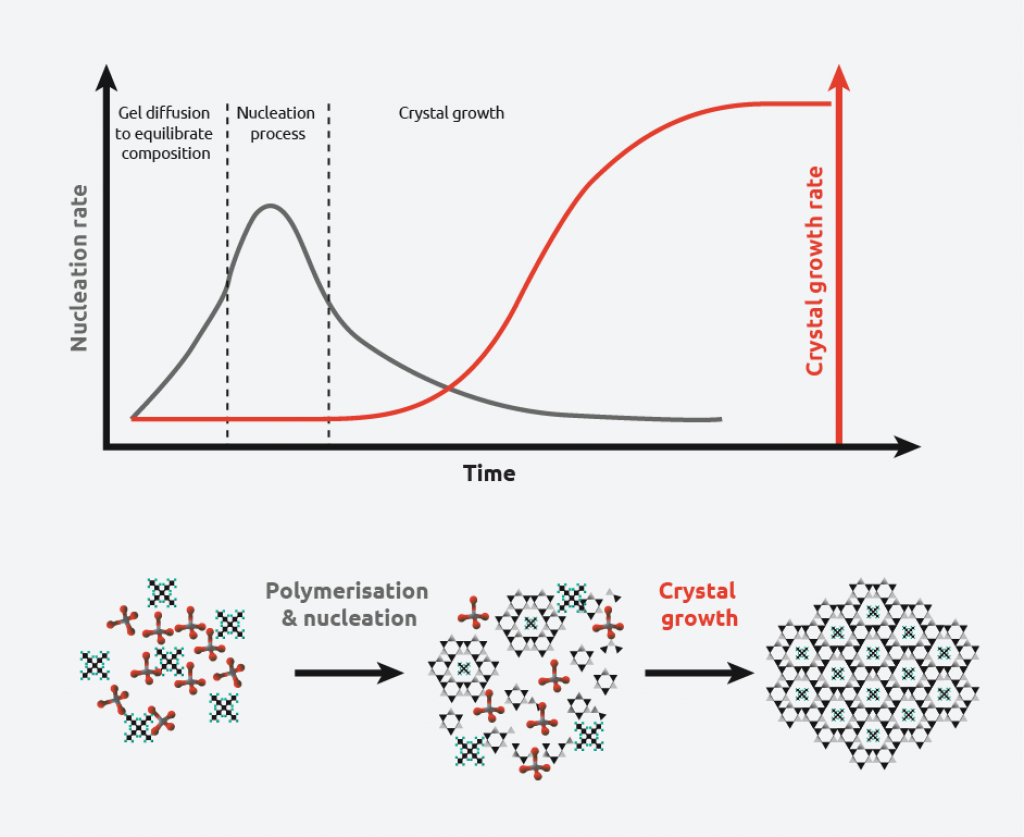
The crystallization process consists of two major events:
- Nucleation – Molecules gather together in clusters in a defined manner. Clusters need to be stable under current experimental conditions to reach the “critical cluster size” or they will redissolve. It is this point in the crystallization process that defines the crystal structure.
- Crystal Growth – Nuclei that have successfully achieved the “critical cluster size” begin to increase in size. Crystal growth is a dynamic process, with atoms precipitating from solution and becoming redissolved. Supersaturation and supercooling are two of the most common driving forces behind crystal formation.
Development of crystallization processes represents a complex and challenging issue, requiring simultaneous control of various product properties, including purity, crystal size and shape, and molecular level solid structure. The control of the nucleation phase is difficult but is the key to process control; crystallization chemists usually aim to achieve goals of high purity and high yield by solely using controlled cooling crystallization techniques.
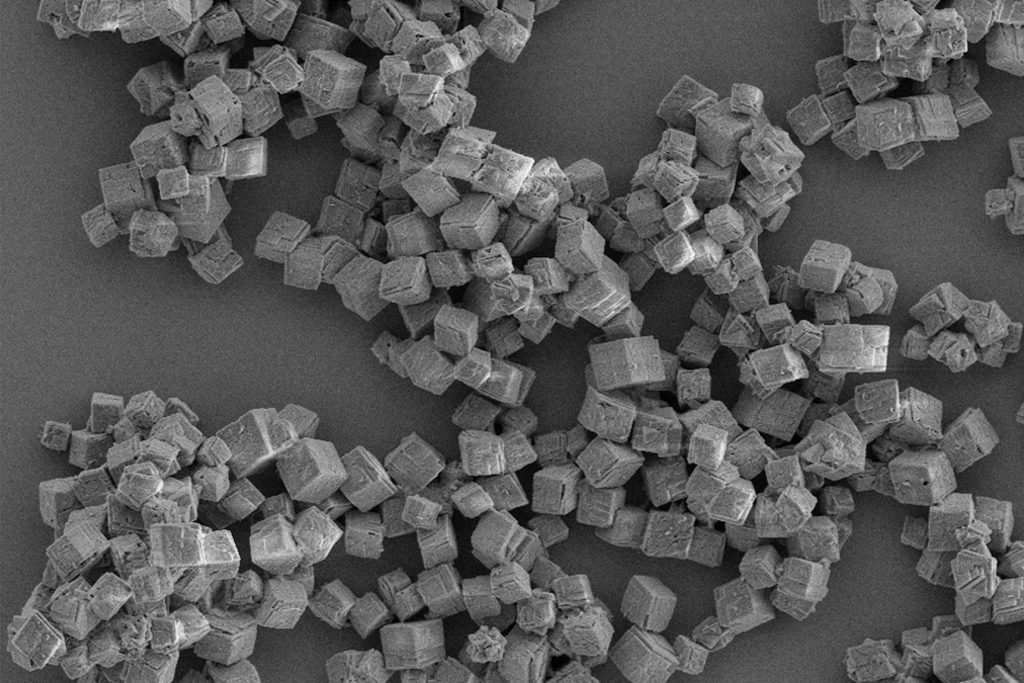
Many compounds can exist in multiple crystal structures – a phenomenon known as “polymorphism” – and can have different physical properties (melting point, shape, dissolution rate, etc.). Depending on the conditions used, either nucleation or crystal growth may be predominant over the other, leading to crystals with different shapes and sizes. Therefore, controlling polymorphism is of significant interest in chemical manufacture.
A common example of the importance of crystal size can be found with ice-cream. Small ice crystals, formed through rapid cooling, improve the texture and taste of the ice-cream compared with larger ice crystals.
Solubility Curves, Supersaturation and the Metastable Zone Width (MSZW)
Traditionally, crystal formation has been achieved by reducing the solubility of the solute in a saturated solution in a variety of ways.
Solubility curves are a common tool used by scientists to understand/demonstrate the relationship between solubility, temperature, and solvent type. By plotting this information, scientists can find the optimum solvent/anti-solvent, temperature, and theoretical yield for their crystallization process.
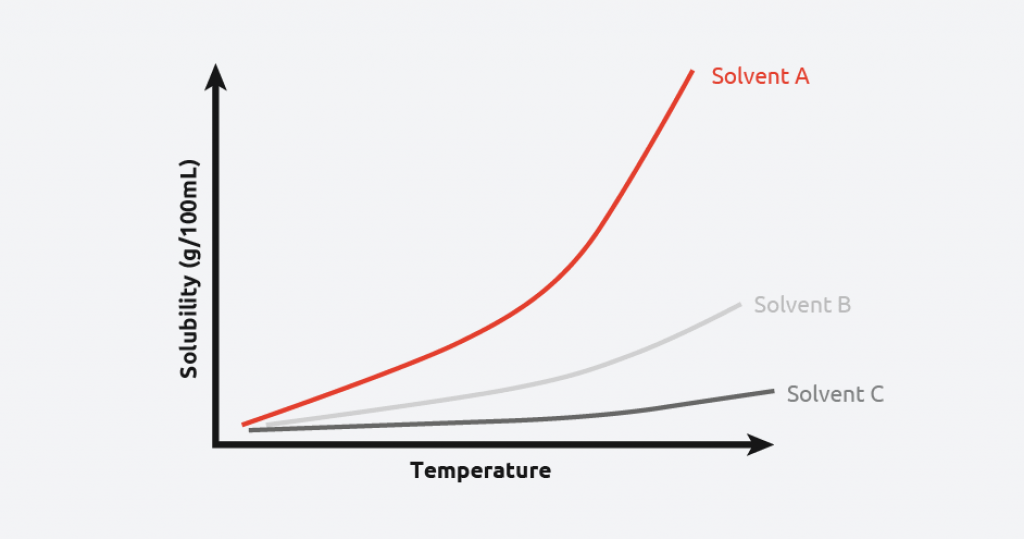
Figure 2 shows that the given material is highly soluble in Solvent A, meaning more material can be crystallized from a given volume of solvent. Conversely, the given material has a low solubility in Solvent C across all temperatures, potentially making it a good anti-solvent for this material
Calculating theoretical crystallization yield
The theoretical yield of crystallization can be calculated at various temperatures:
If a saturated solution containing 45 g of product per 100 g solvent A is cooled from 50 °C to 20 °C, then 15 g of product per 100 g of solvent will remain in solution. Therefore, 30 g of product should crystallize, allowing scientists to measure the yield/efficiency of their crystallization.
In reality, when a saturated solution is cooled, there is more solute in the solution than predicted by the solubility curve, and this is referred to as “supersaturation”.
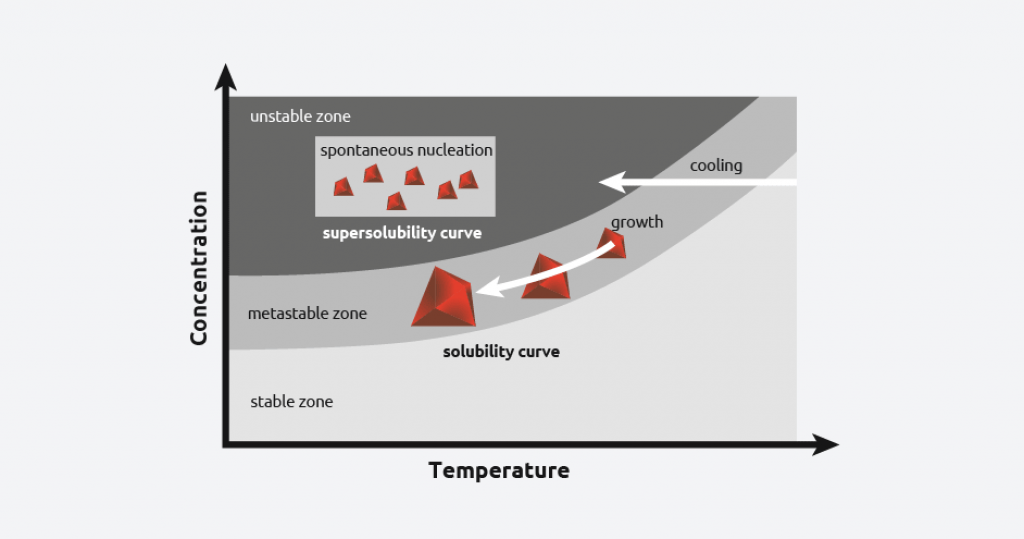
As cooling continues, at a certain temperature, crystal nucleation will begin. This temperature is called the “metastable limit”, and the difference between this temperature and the solubility curve is known as the metastable zone width (MSZW).
By carefully controlling the level of supersaturation of a solution, scientists can control the crystallization process.
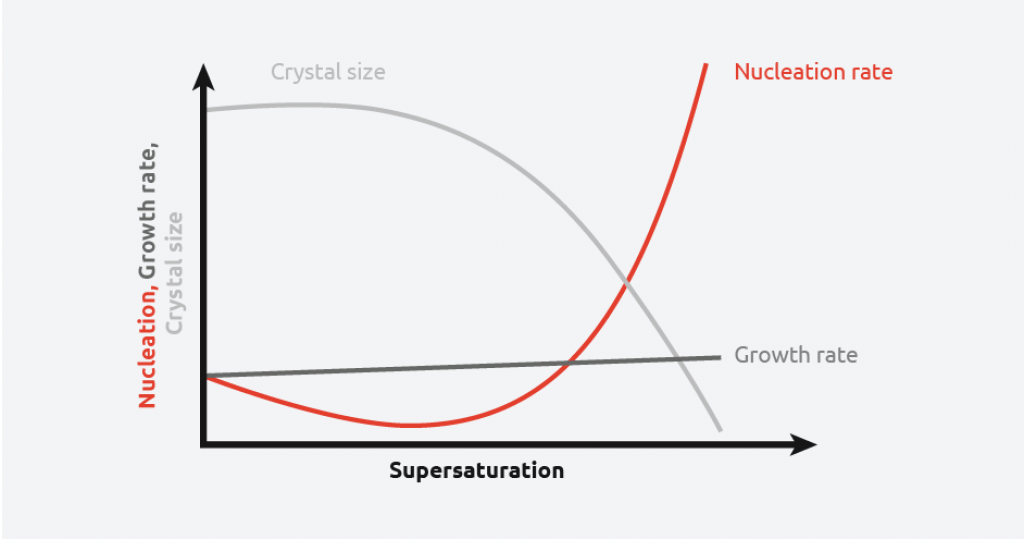
As can be seen from the above schematic, at low levels of supersaturation, crystals grow more quickly than they nucleate resulting in large crystal size distribution. At high supersaturation levels, nucleation dominates crystal growth, providing smaller crystals. This makes understanding and controlling supersaturation vitally important when creating crystals of a desired size and distribution.
What role does crystallization play in industrial research, development, and manufacturing?
Crystallization is one of the most widely used technologies in chemical industry, and process robustness governs process productivity and economics. In particular, the pharmaceutical and food sectors are utilizing crystallization for optimized separation, purification, and solid form selection. For example, crystallization is the most common method of formation of pharmaceutical solids for Active Pharmaceutical Ingredient (API) development. The optimization of the particulate properties such as particle size and shape distributions is paramount as the physical form dictates drug product quality and effectiveness.
Many pharmaceutical drugs have poor physiochemical profiles, such as poor solubility in biological fluids. Significant research and development efforts have been made towards developing a solid form landscape that covers all possible solid structures, including polymorphs, solvates, co-crystals, salts, and the amorphous phase to improve Active Pharmaceutical Ingredient (API) development.
Methods of crystallization
Crystallization is the oldest “unit operation” in a chemical engineering sense. For example, Sodium Chloride has been manufactured this way since the dawn of civilization.
Various traditional methods for crystallization exist, with each technique having unique benefits and drawbacks. The method chosen must be selected based on the properties of the material being crystallized.
- Solvent Evaporation – Easy to set-up, requires air stable samples, requires a minimum solvent volume to work effectively. Large amount of material required.
- Slow Cooling – Requires solvents with boiling points less than 100 °C and moderate solute solubility. Large amount of material required.
- Solvent/Vapour Diffusion – Works well with small amounts of material, however finding two suitable solvents can be challenging. Can “oil out”.
- Sublimation – Not the method of choice for diffraction quality crystals. Typically performed at high temperatures, causing crystals to grow too quickly.
Sonocrystallization
Crystallization processes are often difficult to control, but sonocrystallization is a more modern method of crystallization that offers significant advantages over traditional methods.
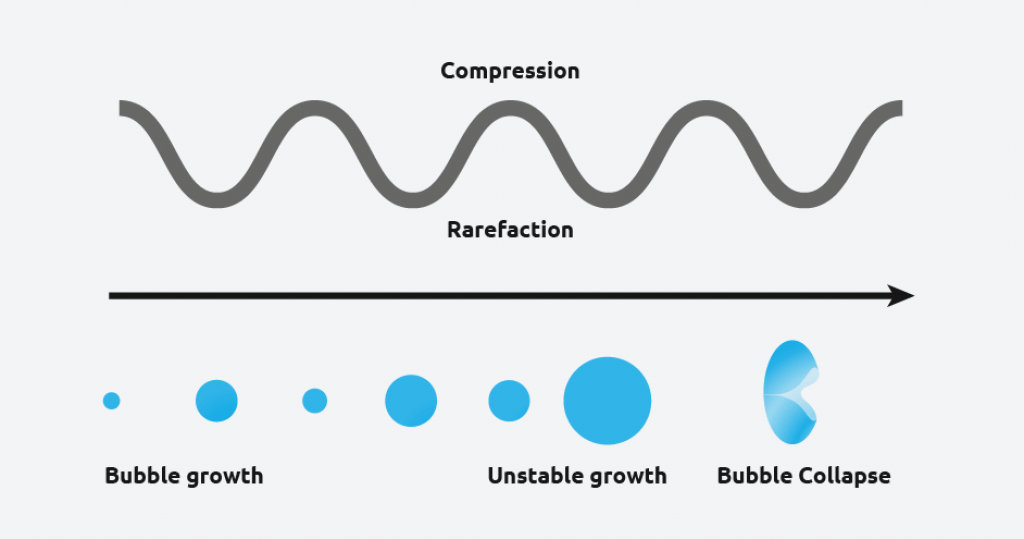
Ultrasound radiation is known to induce acoustic cavitation in liquids through the formation, growth, and collapse of bubbles. The collapse of the bubble provides energy to encourage the nucleation process at the earliest possible point in time. This results in highly repeatable and predictable crystallizations, and offer various benefits, including;
- Reduced induction time
- A decrease in metastable zone width (MSZW)
- Increased nucleation rate
- Increased crystal growth rate
- Reduce agglomeration
- Tailored crystal size distribution
Discover the Sonocrystallization application page for more information
Crystallization monitoring methods
Having decided on a method of crystallization, it is important to measure the progress and subsequent success of your crystallization process.
Measuring crystallization using turbidity
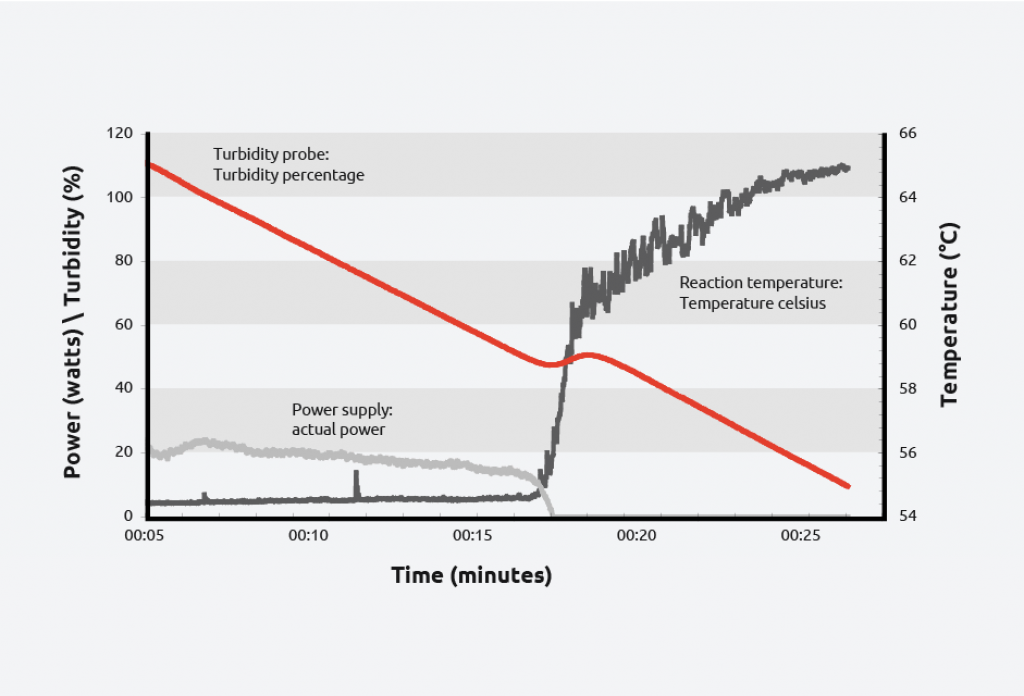
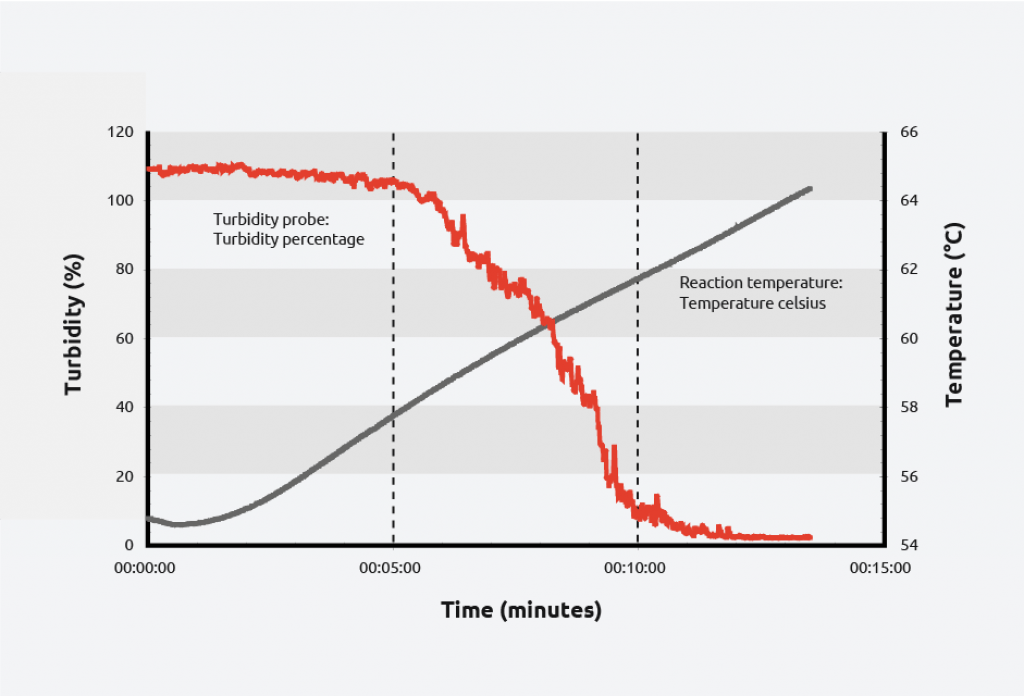
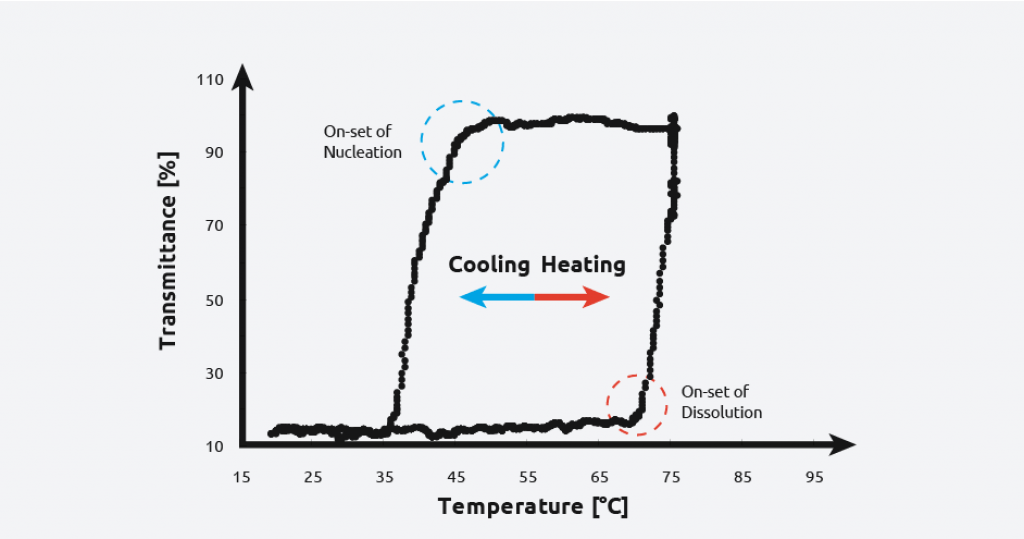
Turbidity probes have been used to monitor crystallizations for decades, due to their ease of use, sensitivity, and affordability. Turbidity probes work by measuring light that is scattered by suspended solids in a liquid. As the total suspended solids increases, the turbidity level (and the cloudiness/haziness) increases, making it a useful tool for calculating the metastable zone of a system and monitoring the formation of crystals.
To create a solubility curve, different concentrations of solute are ramped up and down in temperature, allowing nucleation/dissolution points to be calculated. As an example, turbidity information for the crystallization and dissolution of adipic acid is shown below:
As can be seen on the graph, at a given concentration, adipic acid will spontaneously nucleate at approximately 59 °C and the solution clears at approximately 64 °C giving a metastable zone width of 5 °C.
-
Other methods of crystallization monitoring
A number of alternative probes are also available for crystallization monitoring that provide additional information, such as “chord length” (a fundamental measurement related to particle size), and concentration of solute in the mother liquor or crystal shape/distribution.
FBRM
FBRM probes work by directing a laser beam down the probe, through rotating optics and focussing it at the probe window and measuring the light backscattered by particles in solution.
As the focussed beam scans the solution, individual particles can backscatter the light to the detector. Each pulse of backscattered light is detected and it’s intensity measured. Multiplying the duration of each pulse provides the distance across each particle, known as “chord length”.
Video Imaging
Video imaging probes have also been used determine crystal shape and crystal size distribution, however, at present, they are hard to implement at a commercial scale due to operating temperature limits and the bulky size of such probes.
ATR-FTIR
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) has also been used to gather solubility and supersaturation data, by measuring the concentration of solute in the mother liquor. Additional information can also be gleaned, such as confirmation of the presence of additives or impurities.
-
What technology is available to help chemists perform crystallization monitoring and control?
Chemists have a range of tools available to them for performing, monitoring, and controlling crystallization chemistry. Syrris systems offer a range of solutions to the problem of crystallization monitoring and control.
Crystallization monitoring and control with Atlas HD Crystallization
Atlas HD Crystallization is an intelligent and automated jacketed reactor system that offers a turbidity probe for monitoring the crystallization process, and the innovative SonoLab module to perform sonocrystallization or sonomilling techniques. The system can also integrate a Lasentec FBRM probe for particle size analysis.
Using the Syrris Atlas HD Crystallization system for your crystallization studies offers various benefits, including;
- Fully automated synthesis – Intelligent solvent additions and temperature control are made easy with the Atlas Software allowing easy meta-stable zone analysis
- Sonocrystallization – crystallization with narrow particle size distribution and polymorph control – See the Sonocrystallisation Applications page or the Atlas HD Sonolab system
- Excellent temperature control using sophisticated PID temperature control
- Extremely narrow particle distribution, improved polymorph selectivity, and complete control of nucleation
Syrris products offer a range of solutions to the problem of crystallization control and monitoring.
For more information about crystallization or how you can achieve better results using Syrris products, please contact us.