Crystallization in drug development
Crystallization is a fundamental process in drug development, playing a crucial role in defining the quality, efficacy, and manufacturability of pharmaceutical products. The process of crystallization in the pharmaceutical industry, is employed to purify active pharmaceutical ingredients (APIs) and control their physical and chemical properties. Understanding the mechanisms of crystal growth, nucleation, and the behavior of different crystal forms is essential for developing stable, effective, and high-quality drug products.
This article will explore crystallization in drug development, including its techniques, challenges, applications, and the solutions provided by Syrris that meet the unique needs of the pharmaceutical industry.
What is crystallization?
Crystallization is the process by which a solid form is generated from a solution, melt, or vapor phase. It involves the formation of a crystal lattice where molecules arrange themselves in a highly ordered and repeating structure. The pharmaceutical crystallization process is governed by factors such as temperature, concentration, solvent choice, and other process parameters. In drug development, crystallization is used to purify compounds, define the solid state of APIs, and ensure the right crystalline form is achieved to optimize the drug’s physicochemical properties, including solubility, stability, and bioavailability.
The success of crystallization depends on understanding the interactions between molecules and controlling conditions to promote the desired crystal growth. In pharmaceutical manufacturing, precise control over the crystallization process can lead to improved product quality, yield, and processing efficiency, making it a critical step in the drug development pipeline.
Syrris’ Atlas HD Crystallization Reactor is an ideal solution for the reproducible control of crystallization and sonocrystallization, offering real-time data monitoring in addition.

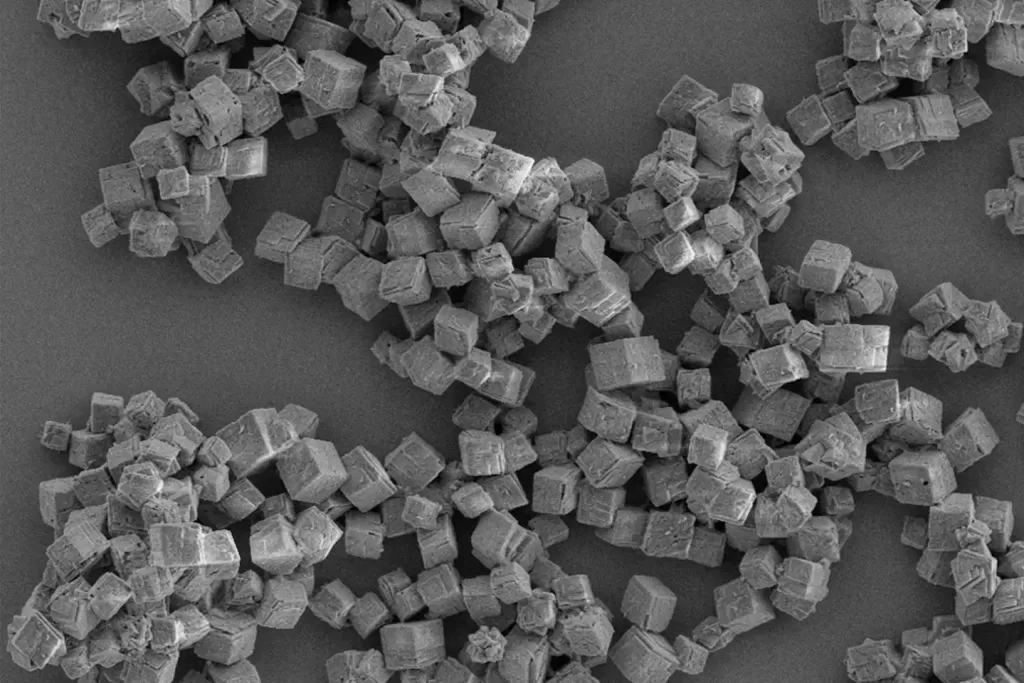
Different crystal forms and their impact on drug development batch methods
Crystal forms, also known as polymorphs, refer to different structural arrangements of the same chemical compound in the solid state. These variations can significantly affect a drug’s physicochemical properties, including solubility, dissolution rate, stability, and bioavailability. For instance, a drug’s dissolution rate is closely linked to its crystal form, impacting how quickly it can be absorbed into the body. Thus, choosing the appropriate crystal form is essential for optimizing the performance of pharmaceutical products.
Different crystalline forms can exhibit varying levels of solubility and stability. For example, a more stable polymorph might have lower solubility, which can limit the drug’s bioavailability. Conversely, a less stable form might dissolve more quickly but could be prone to degradation. Therefore, identifying and selecting the optimal crystal form during the drug development process is a critical step that can influence the efficacy and shelf life of the final product. The pharmaceutical industry invests considerable effort in crystal engineering to identify suitable solid forms and develop scalable crystallization processes to ensure consistent product quality.
Nucleation and crystal growth mechanisms
The crystallization process involves two main stages: nucleation and crystal growth. Nucleation is the initial step where molecules in a solution start to come together to form a stable nucleus, which acts as a seed for further crystal growth. There are two types of nucleation: primary nucleation, which occurs spontaneously without any existing crystals, and secondary nucleation, which happens in the presence of existing crystals and is often induced by mechanical agitation or impurities.
Crystal growth follows nucleation and is the process where the initial nuclei expand to form larger, well-defined crystals. Factors such as temperature, supersaturation, solvent, and additives play a significant role in influencing the rate of crystal growth and the resulting crystal size and morphology. Controlling these factors is crucial for achieving the desired solid form and particle size distribution. The ability to manipulate nucleation and crystal growth mechanisms allows researchers to tailor the properties of the final crystalline product, such as particle size, shape, and purity, which are important for downstream processing and drug performance.
Polymorphism in pharmaceutical development
Polymorphism refers to the ability of a compound to crystallize into more than one crystal structure. This phenomenon can lead to the formation of different solid forms, each with distinct physical and chemical properties. Polymorphs can differ in terms of melting point, solubility, mechanical properties, and stability, which can have significant implications for drug formulation and manufacturing.
During drug development, it is essential to identify and control polymorphism to ensure the safety, efficacy, and quality of the pharmaceutical product. Regulatory guidelines require a thorough understanding of a drug’s polymorphic forms to prevent issues related to bioavailability and stability. Techniques such as X-ray diffraction, thermal analysis, and spectroscopy are commonly used to characterize and study polymorphs. By understanding the conditions that favor the formation of specific polymorphs, researchers can develop robust crystallization processes that produce the desired polymorph consistently, reducing the risk of unexpected changes in the drug substance.
Co-crystallization and its benefits
Co-crystallization is an advanced technique used to improve the physicochemical properties of a drug by forming a crystalline structure composed of two or more components, typically an API and a co-former. Unlike traditional polymorphs, co-crystals are not merely different arrangements of the same molecule but are distinct compounds with a new crystal lattice formed through non-covalent interactions between the components.
Pharmaceutical co-crystals can enhance the solubility, dissolution rate, and stability of poorly soluble drugs, offering a viable solution to formulation challenges. Co-crystallization can also modify other physical properties such as melting point, mechanical behavior, and hygroscopicity, which are important for manufacturing and storage. The selection of suitable co-formers and optimization of crystallization conditions are critical to developing stable co-crystals with desired characteristics. This approach allows for the design of solid forms that provide enhanced drug delivery and performance without altering the molecular structure of the API.
Crystallization techniques and processes
Various crystallization techniques and processes, including industrial crystallization, are employed in the pharmaceutical industry, depending on the desired outcome and the properties of the API. Some of the commonly used techniques include:
- Cooling crystallization: This technique involves reducing the temperature of a saturated solution to induce crystallization. It is widely used for compounds with temperature-dependent solubility.
- Anti-solvent crystallization: This method involves adding a solvent in which the API is poorly soluble, causing the compound to precipitate out of the solution. It is useful for controlling crystal size and morphology.
- Evaporation crystallization: In this process, the solvent is slowly evaporated from the solution, leading to supersaturation and subsequent crystallization. It is often used for compounds that are sensitive to temperature changes.
- Precipitation crystallization: This technique relies on chemical reactions that produce insoluble compounds, resulting in the formation of crystals. It is used when a rapid crystallization process is required.
- Continuous crystallization: A modern approach that allows for the consistent production of crystals by continuously feeding reactants into a crystallizer. It provides better control over process parameters and can improve product quality and scalability.
Each of these techniques has its advantages and limitations, and the choice of method depends on factors such as the API’s solubility profile, desired crystal form, particle size, and production scale. Careful design of the crystallization process and control over variables such as temperature, concentration, and agitation are essential to achieve consistent and reproducible results.
Challenges in crystallization process development
- Control Over Polymorphism: Uncontrolled polymorphic transitions can lead to changes in a drug’s bioavailability, stability, and efficacy. Identifying and selecting the right polymorph is critical to product quality.
- Particle Size Distribution: Achieving uniform particle size distribution is essential for consistent drug formulation and dissolution. Variability in particle size can affect drug performance and manufacturing efficiency.
- Process Scalability: Scaling up the crystallization process from the laboratory to industrial production can introduce challenges related to mixing, heat transfer, and control over process parameters.
- Impurities and Purity Control: Impurities can affect crystal growth, leading to undesirable crystal forms or inconsistent product quality. Effective purification and process control are necessary to ensure high-purity APIs.
- Process Optimization: Fine-tuning parameters such as temperature, solvent, and concentration requires extensive experimentation and data analysis. Advanced modeling and simulation tools can aid in optimizing crystallization processes within pharmaceutical systems to improve efficiency and quality.
Drug discovery and development applications with Syrris
Syrris is a world leader in providing advanced chemical processing solutions, including systems for crystallization development and optimization. Our technologies enable precise control over crystallization parameters, ensuring the production of high-quality APIs with the desired crystal form, size, and properties. From laboratory-scale experimentation to pilot-scale production, Syrris offers a range of customizable crystallization solutions designed to meet the unique needs of the pharmaceutical industry.
Our products are used in laboratories worldwide for a number of applications, like pharmaceutical applications, including drug discovery, process development, and scale-up. Whether you are working on optimizing the crystal growth of a new drug candidate or enhancing the solubility of an existing product through co-crystallization, Syrris provides the tools and expertise to support your research and development efforts.
Syrris batch reactors, such as the Atlas HD Crystallization Reactor, and Orb Jacketed Reactor, are engineered to deliver consistent and reproducible results, making them ideal for batch crystallization processes and ensuring that your crystallization processes are efficient and scalable.
Ready to enhance your crystallization process development? Contact Syrris today to learn more about our innovative solutions for the pharmaceutical industry and how we can help you achieve success in drug development.

Conclusion
Crystallization is a vital process in drug development that directly affects the quality, performance, and manufacturability of pharmaceutical products. Understanding the mechanisms of nucleation, crystal growth, and polymorphism allows researchers to design effective crystallization processes that optimize the solid-state properties of APIs. The use of advanced crystallization techniques, such as co-crystallization and continuous crystallization, can address challenges related to solubility, stability, and particle size, leading to better drug formulations and enhanced patient outcomes.
Syrris provides state-of-the-art solutions for crystallization process development, offering the pharmaceutical industry the tools needed to streamline drug discovery and manufacturing. With precise control over process parameters, Syrris’ Atlas HD Crystallization Reactor and Orb Jacketed Reactor help researchers achieve high-quality, scalable, and reproducible crystallization processes, supporting the production of safe and effective pharmaceutical products.
Contact our team of specialists to find out how Syrris can help you achieve success in drug development.
Syrris products featured in this article

Atlas HD Crystallization Reactor

Orb Jacketed Reactor
What we say
When performing crystallization reactions, the ability to quickly record data is critical. Atlas HD enables this at the touch of a button.”
