Publication – A three step continuous flow synthesis of the biaryl unit of the HIV protease inhibitor Atazanavir
Org. Biomol. Chem. 2013, 11, pp 6806-6813
Luciana Dalla-Vechia,a Benedikt Reichart,b Toma Glasnov,b Leandro S. M. Miranda,a C. Oliver Kappe*b,c and Rodrigo O. M. A. de Souza*a
aBiocatalysis and Organic Synthesis Group, Chemistry Institute, Federal University of Rio de Janeiro, CEP 22941 909, RJ, Brazil.
bChristian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, A-8010 Graz, Austria.
cChemistry Department, Faculty of Science, King Abdulaziz University, P.O. Box 80203, Jeddah 21589, Saudi Arabia
This paper describes a three-step process in flow for the synthesis of a key building block for the drug Atazanavir. The process includes a Suzuki coupling, a hydrazone formation and a hydrogenation. For each step, microwave reaction conditions were transferred to flow and optimised in order to obtain maximum conversion and yield. The use of the Asia FLLEX Module for purifying the reaction mixture enabled to run two of those steps in one sequence. This work is a stepping stone towards the synthesis of Atazanavir in a single continuous flow process.
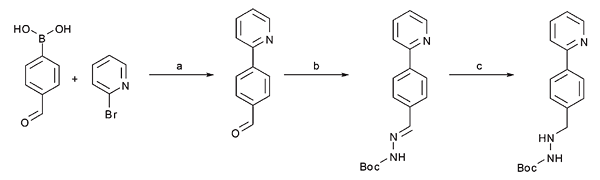
Abstract: The development of multistep continuous flow reactions for the synthesis of important intermediates for the pharmaceutical industry is still a significant challenge. In the present contribution the biaryl-hydrazine unit of Atazanavir, an important HIV protease inhibitor, was prepared in a three-step continuous flow sequence in 74% overall yield. The synthesis involved Pd-catalyzed Suzuki–Miyaura cross-coupling, followed by hydrazone formation and a subsequent hydrogenation step, and additionally incorporates a liquid–liquid extraction step.
This paper uses an Asia flow chemistry system and Asia Syringe Pumps.

