Publication – An Ugi multicomponent azide−alkyne cycloaddition approach for the generation of cyclic peptoids
Journal of Organic Chemistry 2015, 80 (9), pp 4590-4602
- Carlos Eduardo M. Salvador, Bartholomäus Pieber, Philipp M. Neu, Ana Torvisco, Carlos Kleber Z. Andrade, and C. Oliver Kappe
- Institute of Chemistry, University of Graz, Heinrichstrasse 28, A-8010 Graz, Austria
- Laboratório de Química Metodológica e Orgânica Sintética, Instituto de Química, Universidade de Brasília, Campus Universitário Darcy Ribeiro, C.P. 4478, 70904-970, Brasília-DF, Brazil
- Institute of Inorganic Chemistry, Graz University of Technology, Stremayrgasse 9, A-8010 Graz, Austria
Using a Syrris Asia flow chemistry system researchers at the University of Graz and Universidade de Brasilia have demonstrated a complex, multi-step continuous synthesis of cyclic peptoids using a sequential Ugi multicomponent/Cu-catalysed Azide-Alkyne cycloaddition approach.
Peptoids are a class of non-natural oligomeric structure that have applications in drug discovery and possess a wide range of biological activity.
The Ugi four component reaction (U-4CR) is the central transformation in the synthesis and shows the advantages of flow chemistry. The synthesis avoids the handling and exposure of both the potentially explosive azide and the toxic isocyanide intermediates by generating them in-situ.
Using this methodology the group obtained the peptidomimetic core structure in an 80% yield with only a 25min residence time with no purification needed when combining the different transformations.
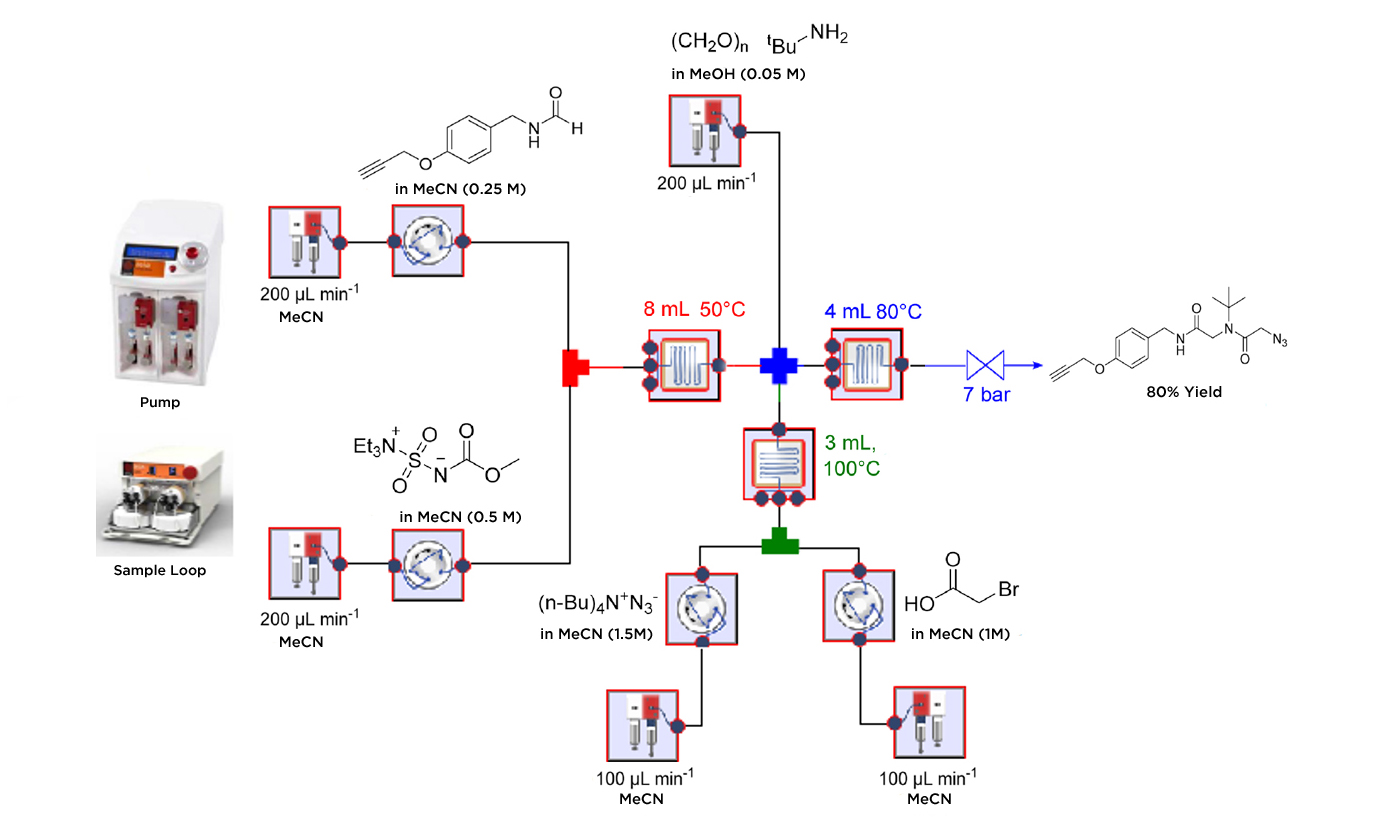
Abstract: The development of a continuous flow multi-step strategy for the synthesis of linear peptoids and their subsequent macrocyclization via Click chemistry is described. The central transformation of this process is an Ugi four-component reaction generating the peptidomimetic core structure. In order to avoid exposure to the often toxic and malodorous isocyanide building blocks, the continuous approach was telescoped by the dehydration of the corresponding formamide. In a concurrent operation, the highly energetic azide moiety required for the subsequent intramolecular copper-catalyzed azide?alkyne cycloaddition (Click reaction) was installed by nucleophilic substitution from a bromide precursor. All steps yielding to the linear core structures can be conveniently coupled without the need for purification steps resulting in a single process generating the desired peptidomimetics in good to excellent yields within a 25 min reaction time. The following macrocyclization was realized in a coil reactor made of copper without any additional additive. A careful process intensification study demonstrated that this transformation occurs quantitatively within 25 min at 140 °C. Depending on the resulting ring strain, either a dimeric or a monomeric form of the cyclic product was obtained.
This paper uses the Asia system. Learn more about the product this chemistry was performed on
For more information, contact us.

