Publication – Expedient Preparation of Nazlinine and a Small Library of Indole Alkaloids Using Flow Electrochemistry
EurJOC 2013, (21), pp 4490-4494
György Kardos and Tibor SoósInstitute of Organic Chemistry, Research Centre for Natural Sciences of the Hungarian Academy of Sciences, 1025 Budapest, Pusztaszeri út 59-67, Hungary
This paper describes the use of immobilised squaramide catalysts for Michael additions. The organocatalysts were packed in glass columns and used on an Asia system in a continuous flow fashion to achieve Michael reactions with a wide range of substrates. Using a solid support version of the catalyst in a flow reactor improved the robustness and simplicity of use of the catalyst.
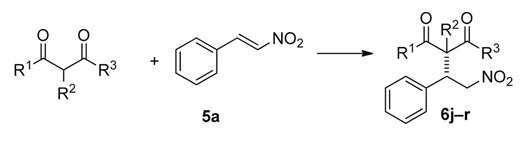
Abstract: This paper describes the preparation of highly efficient, easily accessible, and robust immobilized bifunctional organocatalysts. There was no need to employ any tether to secure high enantio- and diastereoselectivities in various Michael addition reactions. The synthetically useful Michael adducts were obtained within reasonable reaction times with the advantage of easy product isolation and the possibility of automation by using a flow chemistry apparatus.
This paper uses an Asia flow chemistry system.

