Publication – Hydrogen-free alkene reduction in continuous flow
Org. Lett., 2013, 15 (3), pp 710–713
- Andrew S. Kleinke and Timothy F. Jamison
- Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States
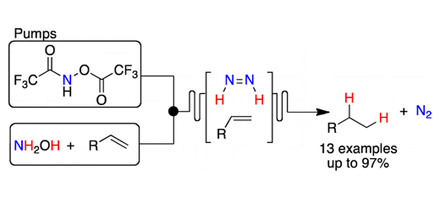
This paper from the prestigious Massachusetts Institute of Technology reports the first continuous flow method for hydrogen free hydrogenation via diimide (diazine, HN=NH) generated in situ by a novel reagent combination.
A mechanistically novel route to the diimide was evaluated via selective O-functionalization (trifluoroacetylation) of hydroxylamine to generate a nitrogen source and amination of a second equivalent of hydroxylamine generated hydrazine oxide, which via elimination of water generated the diimide.
A series of cyclic alkenes, acyclic alkenes and terminal alkynes were optimally reduced giving excellent isolated yields via the diimide reduction route, giving a much safer and cheaper route into alkanes as Hydrogen and Transition metals are not required.

Reaction setup: (a) reagent solutions; (b) syringe pumps; (c) T-shaped mixer (i.d. = 500 μm);
(d) PFA tubing reactor (i.d. = 760 or 1,000 μm); (e) bpr (40 or 100 psi); (f) sample collection flask.
Abstract: The first continuous hydrogenation that requires neither H2 nor metal catalysis generates diimide by a novel reagent combination. The simple flow reactor employed minimizes residence time by enabling safe operation at elevated temperature.
This paper uses an Asia flow chemistry system.

