Publication – Nickel nanocrystals – fast synthesis of cubes, pyramids, and tetrapods
RSC Adv. 2013, 3, pp 1380-1387
- Meital Shviro and David Zitoun
- Bar Ilan University, Department of Chemistry and Bar Ilan Institute of Nanotechnology and Advanced Materials (BINA),
Ramat Gan, 52900, Israel.
In this paper, the authors study the formation and growth of nickel nanocrystals / nanoparticles using organometallic Ni(η4-C8H12)2 as a precursor. The reaction temperature has been identified as the key parameter which determines the shape of the obtained nanoparticles.
In addition to the three classic shapes of nanoparticles (cubical, spherical, pyramidal), a new shape of nanoparticles – tetrapods – has been reported. This shape has only been observed in Syrris microreactors because of the excellent heat transfer and temperature control obtained in those devices.

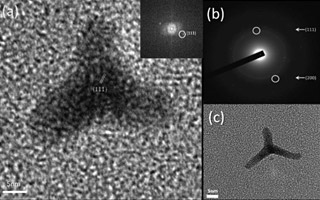
Figure(s): TEM images of synthesis in microfluidic cells
Abstract: We report the use of organometallic precursors in microwave and microfluidic reactors to synthesize Ni nanoparticles of various shapes: spheres, cubes, trigonal pyramids and tetrapods. Each route has the ability to yield a preferential shape based on kinetic control of crystal growth. Tetrapods are obtained only in a microfluidic reactor. Trigonal pyramids crystallize in the metastable hexagonally close-packed (HCP) phase while spheres, cubes and tetrapods crystallize in the thermodynamically stable face-centred cubic (FCC) phase. The magnetic properties of nanocubes (in particular the magnetic coercive field) are found to be greatly enhanced compared to bulk and previous examples of nanostructured Ni materials.
This paper uses an Asia flow chemistry syringe pump and a glass microreactor.

