“Flow chemistry”, sometimes referred to as “plug flow” or “continuous flow chemistry” is the process of performing chemical reactions in a tube or pipe. Reactive components are pumped together at a mixing junction and flowed down a temperature-controlled pipe or tube. This provides some major advantages such as faster reactions, cleaner products, safer reactions and easy scale-up.
What are the benefits of flow chemistry?
-
Faster reactions
Flow reactors are easily pressurized (e.g. Asia systems can be pressurized to 300psi). This allows reactions to be heated 100-150ºC above their normal boiling point, therefore, creating reaction rates that are 1000s of times faster. This process is called superheating.
-
Cleaner products
Flow reactors enable excellent reaction selectivity. The rapid diffusion mixing avoids the issues found in batch reactors. The high surface area to volume ratio (1000x greater than a batch reactor) enables almost instantaneous heating or cooling and therefore ultimate temperature control.
-
Safer reactions
Flow chemistry allows only a small amount of hazardous intermediate to be formed at any instant. The high surface area also allows excellent control of exotherms.
-
Integrated synthesis, work-up, and analysis
Reaction products exiting a flow reactor can be flowed into a flow aqueous workup system or solid phase scavenger column. From there they can be analyzed either in line (e.g. FTIR) or a sample taken, using a sampler and diluter then and injected onto and LCMS.
-
Rapid reaction optimization
Flow Chemistry with automation enables the quick variation of reaction conditions on a very small scale e.g. 100 µL. Parameters such as reaction time, temperature, ratio of reagents, concentration and reagents themselves can all be rapidly varied. One reaction can follow another, separated by solvent, each cleaning out the previous reaction.
-
Easy scale-up
Scale-up issues are minimized due to maintaining excellent mixing and heat transfer. Higher flow rates and correspondingly larger reactors can be used to easily produce kilogram quantities.
-
Reaction conditions not possible using traditional batch chemistry methods
Flow chemistry facilitates reaction conditions not possible in batch such as a 5-second reaction at 250 ºC. Multi-step procedures such as a rapid low-temperature deprotonation followed instantaneously by the addition of an electrophile high temperature are made easy.
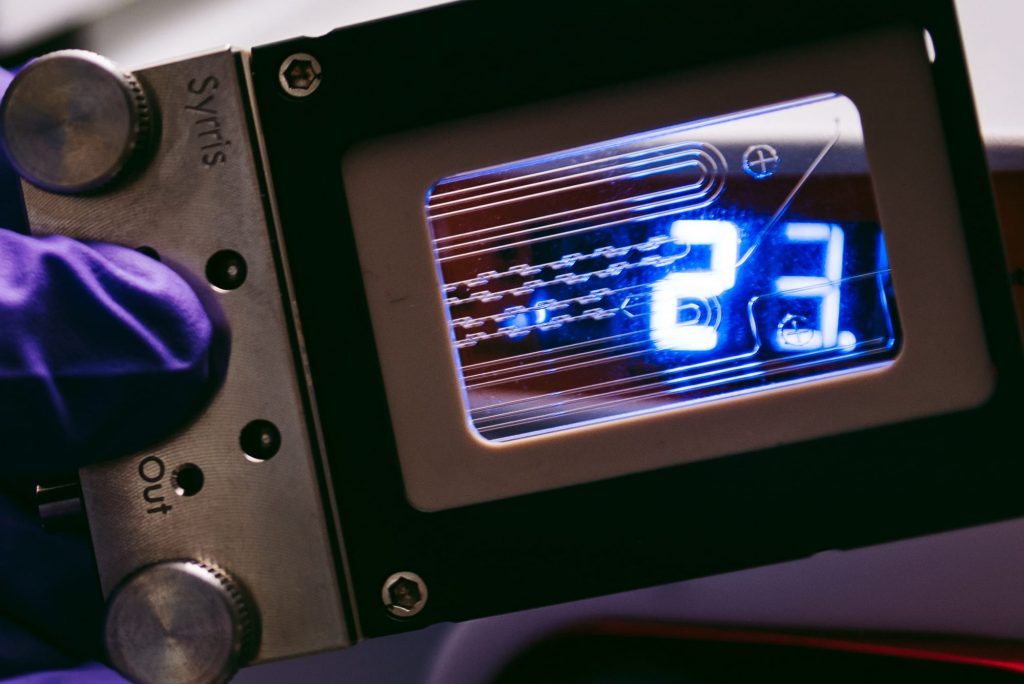
Examples of flow chemistry
Syrris has a range of resources that demonstrate a variety of flow chemistry application notes and reactions using Syrris’ flow chemistry systems. Syrris’ innovative microreactor-based systems include the modular Asia product range. Here are two examples shown below:
-
Oxidation of a primary alcohol
 This paper describes reaction conditions for the oxidation of alcohols in continuous flow using a column reactor packed with polymer-supported tetra-N-alkylammonium perruthenate.
This paper describes reaction conditions for the oxidation of alcohols in continuous flow using a column reactor packed with polymer-supported tetra-N-alkylammonium perruthenate.Chemistry
Organic SynthesisAuthor
Steven V. Ley, Ian R. Baxendale, Jon Deeley, Charlotte M. Griffiths-Jones, Steen Saaby, Geoffrey K. Tranmer (University of Cambridge)System
Syrris Asia -
Williamson Ether Synthesis
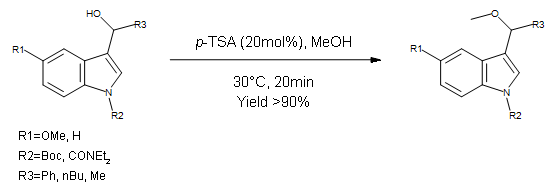
This paper describes a multi-step formation of functionalized indoles. This step focuses on a high yielding Williamson ether synthesis using Methanol as a nucleophile. Chemistry
Organic SynthesisAuthor
Thomas Tricotet and Donal O’Shea (University College Dublin)System
Syrris Asia
Flow chemistry customer stories
A new dimension in drug discovery for Gedeon Richter
The pharmaceutical industry has been leading the way in its use of flow chemistry technology for research and development and manufacturing of new drugs, and Gedeon Richter is no exception. Using a Syrris Asia Flow Chemistry system, researchers have been able to create new heterocyclic scaffolds – chemistry that was impossible to them before adopting flow chemistry techniques.
We purchased an Asia flow chemistry reactor in June 2012, and are reaping the benefits of using flow chemistry techniques. The system has extended the range of chemistries available to us, allowing us to work at much higher pressures and temperatures – sometimes above a solvent’s boiling point – to create completely new heterocyclic scaffolds.”
Precise control of reactions conditions for quantum dot synthesis
Researchers at the University of Cambridge have been using the Asia flow chemistry system to synthesize quantum dots for next-generation solar cells and LEDs.
The growth of quantum dots is susceptible to minute changes in reaction conditions, the unique control that flow chemistry permits gave me precise and repeatable quantum dot growth.”

Flow chemistry publications
The Syrris Asia flow chemistry system has been used in many ground-breaking flow chemistry studies by leading research groups and companies. Discover a selection of papers below, or see the full list here.
-
Development of a continuous flow sulfoxide imidation protocol using azide sources under superacidic conditions
This paper published by Oliver Kappe’s group from the University of Graz, in collaboration with Lonza and AstraZeneca, focuses on an efficient flow protocol for the synthesis of sulfoximine. The imidation of sulfoxides using azides and concentrated sulfuric acid is a high-yielding process for synthesizing sulfoximines but the hazard risks associated with it renders it unsuitable for batch production. It is however perfectly suited for a continuous flow process.
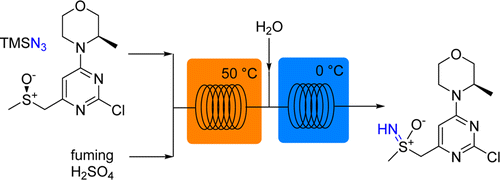
First published: 23rd July 2015 | https://doi.org/10.1002/cssc.201500850
Bernhard Gutmann, Petteri Elsner, Anne O’Kearney-McMullan, William Goundry, Dominique M. Roberge, and C. Oliver Kappe
Organic Process Research & Development 2015 19 (8), 1062-1067
This publication uses an Asia flow chemistry system and the FLUX module
-
Continuous-flow synthesis of regioregular poly(3-Hexylthiophene): Ultrafast polymerization with high throughput and low polydispersity index
This paper describes a continuous process for the synthesis of poly(3-hexylthiophene). The authors have improved on the traditional batch Grignard Metathesis polymerization reaction by identifying a solvent which dissolves the catalyst. The optimized liquid flow process has been successfully run on a Syrris Asia system. The ultra-fast mixing achieved on a Micromixer Chip and the ability to carry the synthesis at high monomer concentrations enable high batch to batch reproducibility, high throughput and very low polydispersity of the obtained material.
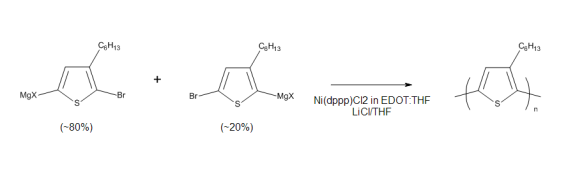
First published online: 15th July 2014 | https://doi.org/10.1556/JFC-D-14-00009
This publication uses an Asia flow chemistry system
-
The methoxylation of N-formylpyrrolidine in a microfluidic electrolysis cell for routine synthesis
This paper describes the application development of a high-yielding, high-throughput N-formyl-pyrrolidine methoxylation reaction carried out in a flow electrochemical cell. The real throughput of the flow electrochemical cell for this specific reaction is also shown to be at least double that of the nearest competitor and has been measured rather than predicted. The publication concludes that the flow electrochemical cell will allow a range of electrochemical syntheses to be routinely carried out in flow, and to enable access to chemical space that was previously unavailable for the non-electrochemistry specialized chemist. This paper uses the Asia Flow Chemistry System and the FLUX Electrochemistry Module.
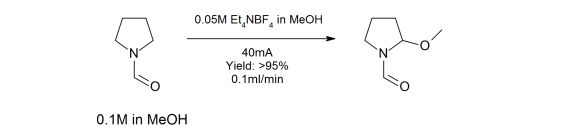
First published: 1st May 20125 | https://doi.org/10.1002/cssc.201500850
Jekaterina Kuleshovaa, Joseph T. Hill-Cousins, Peter R. Birkin, Richard C. D. Brown, Derek Pletcher, Toby J. Underwood
Electrochimica Acta Volume 69, 1 May 2012, Pages 197–202number





